How To Fix Your Circadian Clock
Shift workers, especially those that work all night and sleep during the day, have long since been associated with many cardiovascular diseases like hypertension, diabetes, obesity and even sleep disorders.1 Many of the associated risks arise from a disrupted circadian rhythm and our well-oiled biological clocks. But, our disturbed circadian cycles also affect our vision and make us susceptible to ocular diseases. In my previous blogs, I detailed how retinal signs are early indicators of hypertension, stroke and cardiovascular disorders. Here, I decided to highlight some recent findings that connected vascular complications to our lifestyle choices, vis à vis circadian rhythm.
One of the most photosensitive cells in the retina are the rods and the cones, receptors of light, because of which the eye is also under regulation by the circadian clock – day and night. The master regulator of this clock is in the brain called suprachiasmatic nuclei (SCN), that operate after receiving light through the retina. At the molecular level, not surprisingly, researchers have observed different clock regulating proteins across different cell types throughout the retina. Although research is currently underway and it is still not clearly understood, how the retina is affected by disruptions in the light/dark cycle.
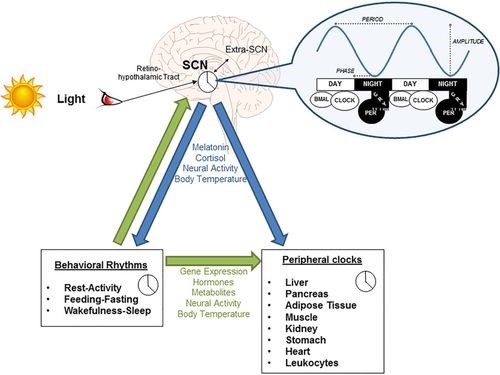
Source: Covassin et al., Hypertension. 2016;68:1081–1090
The figure illustrates the control of SCN by light and how this at the molecular level affects clock protein genes like CLOCK, BMAL and in turn affects peripheral and behavioral patterns.2
One study tried to look at whether one of the clock proteins that was associated with diabetes, had similar effects in the retina. They saw in their animal model, changes in the retina as well as bone marrow, comparable to those seen in diabetic animals.3 Not only this, advanced form of diabetic retinopathy, that is usually caused by abnormally growing blood vessels in the retina, show a reduced expression of clock protein genes in experimental models.4 Another study reported significant changes in the cones of mice with dysfunctional clock gene.5
Many research studies and review articles have documented the effects of disrupted circadian cycles in research settings and shown a correlation. However, direct evidence or causation factors is still under-studied in human patients – calling attention to a gap that beckons researchers to fill this void.
References:
- Covassin et al., Keeping up with the clock. Hypertension. 2016;68:1081–1090
- Liu et al., Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS One. 2012;7(11):e50602
- Bhatwadekar et al., Per2Mutation Recapitulates the Vascular Phenotype of Diabetes in the Retina and Bone Marrow. Diabetes. 2013 Jan; 62(1): 273–282.
- Busik et al., Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. Exp Med. 2009 Dec 21; 206(13):2897-906.
- Ait-Hmyed et al., Mice lacking Period 1 and Period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur J Neurosci. 2013 Apr; 37(7):1048-60.

Trupti Shetty is a PhD Candidate from the Dept. of Pharmacology & Toxicology at Indiana University. She works for Tim Corson in the Glick Eye Institute, studying ocular angiogenesis and she is interested in understanding the molecular pathways of blinding neovascular eye diseases. She tweets as @trups263 and likes connecting with science communicators around the globe. Be wary of her obsession with the Beatles and tendency to like funny dog videos. @trups263
How To Fix Your Circadian Clock
Source: https://earlycareervoice.professional.heart.org/circadian-clock-and-retina/
Posted by: hirschthang1994.blogspot.com

0 Response to "How To Fix Your Circadian Clock"
Post a Comment